Decoding The Quantum World: A Complete Information To Quantum Numbers And Their Chart
By admin / September 17, 2024 / No Comments / 2025
Decoding the Quantum World: A Complete Information to Quantum Numbers and Their Chart
Associated Articles: Decoding the Quantum World: A Complete Information to Quantum Numbers and Their Chart
Introduction
On this auspicious event, we’re delighted to delve into the intriguing matter associated to Decoding the Quantum World: A Complete Information to Quantum Numbers and Their Chart. Let’s weave attention-grabbing data and supply contemporary views to the readers.
Desk of Content material
Decoding the Quantum World: A Complete Information to Quantum Numbers and Their Chart

The seemingly chaotic dance of subatomic particles is ruled by a surprisingly elegant algorithm, encapsulated in a framework of quantum numbers. These numbers, not simply arbitrary labels, present a exact description of an electron’s state inside an atom, revealing its vitality, spatial orientation, and intrinsic angular momentum. Understanding quantum numbers is essential to greedy the construction of atoms, the periodic desk, and the very nature of matter. This text serves as a complete information to quantum numbers, exploring their particular person meanings, interrelationships, and their illustration in a quantum numbers chart.
The 4 Fundamental Quantum Numbers:
The quantum mechanical mannequin of the atom makes use of 4 principal quantum numbers to explain the state of an electron:
-
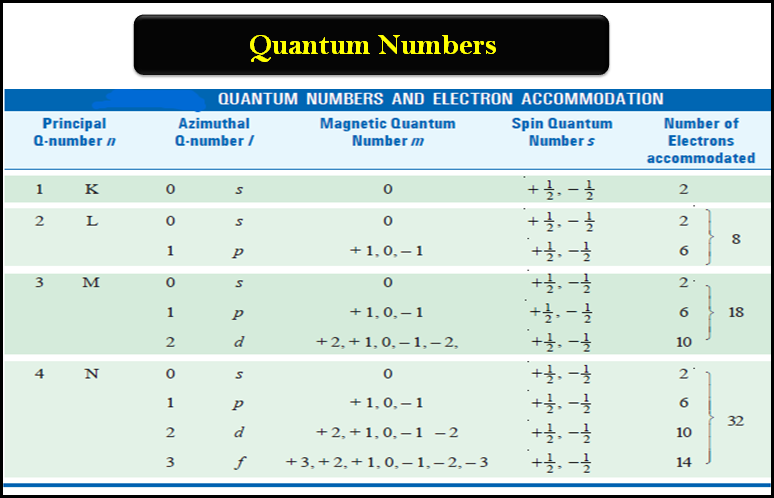
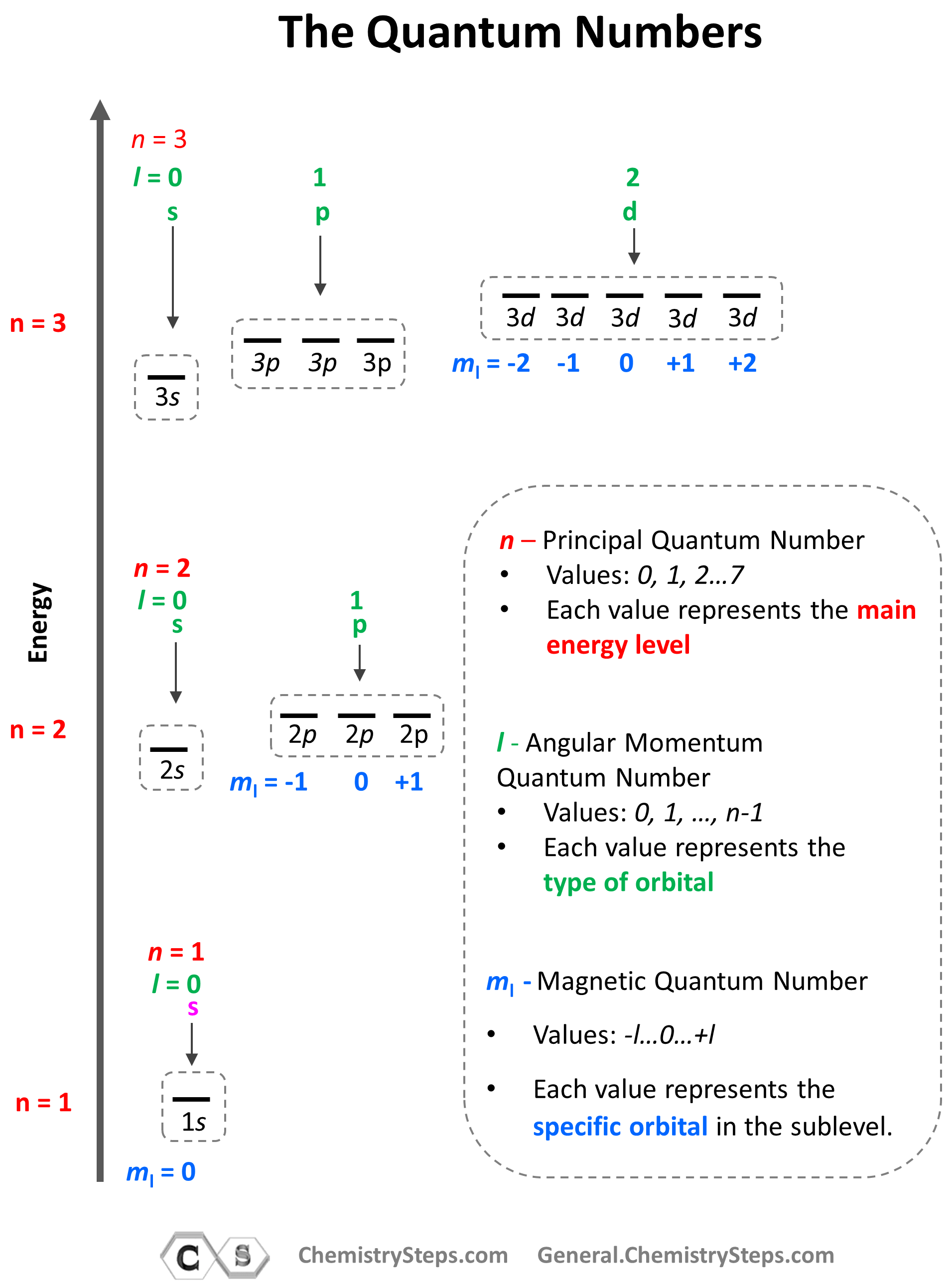
Principal Quantum Quantity (n): That is essentially the most basic quantum quantity, representing the electron’s vitality stage and common distance from the nucleus. It will probably tackle optimistic integer values (n = 1, 2, 3, …). Increased values of ‘n’ correspond to increased vitality ranges and bigger orbitals, which means the electron is farther from the nucleus and fewer tightly certain. The shell, or vitality stage, is straight recognized by ‘n’. For instance, n=1 represents the primary shell (Ok shell), n=2 the second shell (L shell), and so forth. The variety of orbitals in a shell is n².
-
Azimuthal Quantum Quantity (l): Often known as the orbital angular momentum quantum quantity, ‘l’ describes the form of the electron’s orbital and its angular momentum. For a given worth of ‘n’, ‘l’ can tackle integer values from 0 to n-1. Every worth of ‘l’ corresponds to a particular subshell:
- l = 0: s subshell (spherical orbital)
- l = 1: p subshell (dumbbell-shaped orbitals)
- l = 2: d subshell (extra complicated shapes)
- l = 3: f subshell (much more complicated shapes)
- and so forth…
The variety of orbitals inside a subshell is given by 2l + 1. The subshells are designated by letters (s, p, d, f) as a substitute of numbers for historic causes. The angular momentum related to a subshell is proportional to √(l(l+1))ħ, the place ħ (h-bar) is the diminished Planck fixed.
-
Magnetic Quantum Quantity (ml): This quantity specifies the orientation of the orbital in house relative to an exterior magnetic discipline. For a given worth of ‘l’, ‘ml’ can tackle integer values from -l to +l, together with 0. For instance, if l = 1 (p subshell), ml might be -1, 0, or +1, representing three completely different p orbitals oriented alongside the x, y, and z axes, respectively. This spatial quantization demonstrates that the angular momentum vector can solely tackle particular discrete orientations.
-
Spin Quantum Quantity (ms): This intrinsic property of the electron describes its spin angular momentum, an inherent property unrelated to its orbital movement. It will probably solely tackle two values: +1/2 (spin up, denoted by ↑) or -1/2 (spin down, denoted by ↓). That is typically visualized because the electron spinning on its axis, though it is a classical analogy that should not be taken actually. The spin angular momentum is given by ±(1/2)ħ. The Pauli Exclusion Precept states that no two electrons in an atom can have the identical set of 4 quantum numbers; due to this fact, every orbital can maintain a most of two electrons with reverse spins.
The Quantum Numbers Chart:
A quantum numbers chart systematically organizes the doable values of those 4 quantum numbers for a given principal quantum quantity (n). It supplies a concise overview of the electron configuration inside an atom. The chart usually presents the values of n, l, ml, and ms for every electron within the atom. Whereas a full chart for a posh atom can be intensive, we are able to illustrate the precept with examples:
| n | l | ml | ms | Orbital Designation | Most Electrons |
|---|---|---|---|---|---|
| 1 | 0 | 0 | +1/2, -1/2 | 1s | 2 |
| 2 | 0 | 0 | +1/2, -1/2 | 2s | 2 |
| 2 | 1 | -1, 0, +1 | +1/2, -1/2 (every ml) | 2p | 6 |
| 3 | 0 | 0 | +1/2, -1/2 | 3s | 2 |
| 3 | 1 | -1, 0, +1 | +1/2, -1/2 (every ml) | 3p | 6 |
| 3 | 2 | -2, -1, 0, +1, +2 | +1/2, -1/2 (every ml) | 3d | 10 |
This simplified chart reveals the quantum numbers for the primary three shells (n=1, 2, 3). Every row represents a particular orbital, characterised by its distinctive set of n, l, and ml. The ms column signifies the doable spin states for every orbital. The "Orbital Designation" column combines the n and l values to supply a concise label (e.g., 1s, 2p, 3d). The "Most Electrons" column reveals the utmost variety of electrons that may occupy every subshell (or orbital).
Purposes of Quantum Numbers:
The understanding and utility of quantum numbers are basic to varied features of chemistry and physics:
-
Electron Configuration and the Periodic Desk: Quantum numbers clarify the association of electrons in atoms, which straight pertains to the periodic desk’s construction and the chemical properties of components. The filling of orbitals in line with the Aufbau precept (filling orbitals so as of accelerating vitality) and Hund’s rule (maximizing unpaired electrons in a subshell) is dictated by the quantum numbers.
-
Spectroscopy: The absorption and emission of sunshine by atoms are straight associated to the vitality variations between electron orbitals, that are decided by the principal quantum quantity. Spectroscopic methods analyze the emitted or absorbed gentle to find out the digital construction of atoms and molecules.
-
Molecular Orbital Principle: Quantum numbers are additionally important in understanding the formation of chemical bonds in molecules. The mix of atomic orbitals to type molecular orbitals is ruled by the symmetry and vitality ranges of the atomic orbitals, described by quantum numbers.
-
Nuclear Physics: Whereas the 4 quantum numbers primarily describe electrons, related quantum numbers are used to characterize the states of nucleons (protons and neutrons) inside the atomic nucleus. These numbers assist perceive nuclear construction, stability, and radioactive decay.
Past the Fundamentals:
Whereas the 4 principal quantum numbers present a strong framework for understanding atomic construction, extra refined quantum numbers are needed for a whole description of complicated techniques. These embrace:
-
Spin-orbit coupling: This interplay between an electron’s spin and its orbital angular momentum results in a high-quality construction in atomic spectra, requiring extra quantum numbers to explain the coupled states.
-
Relativistic results: At excessive atomic numbers, relativistic results turn into important, influencing the vitality ranges and requiring changes to the quantum quantity framework.
Conclusion:
Quantum numbers present a strong and stylish framework for understanding the complicated world of atomic construction. The quantum numbers chart, though probably intensive for bigger atoms, serves as an important device for visualizing and deciphering the digital configuration of atoms and molecules. The rules outlined listed below are basic to quite a few areas of chemistry, physics, and supplies science, highlighting the profound impression of quantum mechanics on our understanding of the universe. Additional exploration into the intricacies of quantum mechanics reveals a deeper appreciation for the delicate but highly effective guidelines governing the conduct of matter on the atomic stage.




Closure
Thus, we hope this text has offered invaluable insights into Decoding the Quantum World: A Complete Information to Quantum Numbers and Their Chart. We admire your consideration to our article. See you in our subsequent article!